
The Kerkermeister Pension | Auerbach, Vogtland
Section: Travel
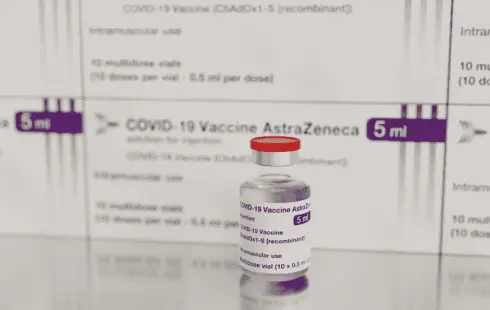 Following the authorization of the coronavirus vaccine developed by the British-Swedish company Astrazeneca, concerning reports emerged. The vaccine, known as "Vaxzevria," was associated with severe side effects in extremely rare instances. Some individuals vaccinated experienced thrombosis in the brain or abdominal organs, resulting in fatalities. Initially believed to predominantly affect young women, the vaccine was initially advised only for individuals over 60 in Germany and later not recommended at all.
Following the authorization of the coronavirus vaccine developed by the British-Swedish company Astrazeneca, concerning reports emerged. The vaccine, known as "Vaxzevria," was associated with severe side effects in extremely rare instances. Some individuals vaccinated experienced thrombosis in the brain or abdominal organs, resulting in fatalities. Initially believed to predominantly affect young women, the vaccine was initially advised only for individuals over 60 in Germany and later not recommended at all.
Questions arose regarding what Astrazeneca knew about these occurrences and whether they could have been prevented. A woman from Upper Franconia, who suffered from thrombosis in the intestine and fell into a coma after receiving the Astrazeneca vaccine in March 2021, demanded not only compensation from the pharmaceutical company but also the disclosure of documents from the clinical trials.
A partial victory was achieved by the woman before the Bamberg Higher Regional Court. On Monday, a civil chamber ruled that the company must provide comprehensive information regarding all known effects and side effects of its coronavirus vaccine, specifically those relevant to the plaintiff's clinical condition. This encompassed any additional findings gathered between the vaccine's approval in December 2020 and February 2024.
The clinical condition of the plaintiff is referred to as "thrombosis with thrombocytopenia syndrome" (TTS). This severe thrombosis, occurring extremely rarely not only after vaccinations with Astrazeneca's Covid vaccine but also with the similar vaccine from Johnson & Johnson, is accompanied by a decrease in blood platelets. It appears to stem from antibodies formed post-vaccination attacking the blood platelets. Both Astrazeneca's and Johnson & Johnson's Covid vaccines are based on modified cold viruses.
The frequency of TTS following vaccination with these vector vaccines is not precisely known but is established to be extremely rare. As of early April 2021, the European Medicines Agency had identified 169 cases of cerebral vein TTS and 53 cases of abdominal TTS among 34 million vaccinated individuals. Initially presumed to be more prevalent in younger individuals and women, it is now believed to affect the entire population uniformly.
The plaintiff's lawyer, Volker Loeschner, expressed satisfaction with the court's decision, anticipating further requests for information in similar cases. This marked the first trial of its kind in Germany.
A court spokesperson clarified that the disclosed information pertained solely to the woman's illness and was only required to be made available to her. While the woman sought information on other side effects, the chamber declined this request, with no avenue for appeal.
Meanwhile, the lawsuit for damages and compensation for pain and suffering persists. Astrazeneca's legal representatives have thus far rejected the possibility of a settlement with the plaintiff, citing the decision of the Hof Regional Court. In the initial instance, the court dismissed the woman's lawsuit due to an absence of identified product defects or information errors related to the vaccine.
A company spokesperson stressed that Astrazeneca consistently adhered to regulatory standards and emphasized the continued positive benefits of vaccination. "Regulatory authorities worldwide have affirmed that the benefits of vaccination with our Covid-19 vaccine Vaxzevria outweigh the risks of the extremely rare potential side effects," the spokesperson stated.
Image by Paul McManus from Pixabay

Section: Travel

Section: Arts

Section: Arts

Section: Arts

Section: Fashion

Section: Politics

Section: Fashion

Section: News

Section: Business

Section: Fashion
Both private Health Insurance in Germany and public insurance, is often complicated to navigate, not to mention expensive. As an expat, you are required to navigate this landscape within weeks of arriving, so check our FAQ on PKV. For our guide on resources and access to agents who can give you a competitive quote, try our PKV Cost comparison tool.
Germany is famous for its medical expertise and extensive number of hospitals and clinics. See this comprehensive directory of hospitals and clinics across the country, complete with links to their websites, addresses, contact info, and specializations/services.
Join us at the Kunstraum in der Au for the exhibition titled ,,Ereignis: Erzählung" by Christoph Scheuerecker, focusing on the captivating world of bees. This exhibition invites visitors to explore the intricate relationship between bees and their environment through various artistic expressions,...



No comments yet. Be the first to comment!